

Scientists have long been uncertain how a particular bacterium, responsible for many cases of ulcers, can survive in such an inhospitable environment as the human stomach—a place with a pH somewhere between that of lemon juice and battery acid.
But LAS researchers have now begun to reveal answers that may have implications for drug treatments.
About half of the world’s population is infected with Helicobacter pylori, although most infected people do not know it and have no obvious symptoms. H. pylori is behind most human cases of gastric and duodenal ulcers, and long-term infection is a significant risk factor for stomach cancer, the second leading cause of cancer death worldwide.
“Even though H. pylori is a common resident of the human stomach, the bug is, in and of itself, not particularly well adapted to this harsh environment,” says Steven Blanke, a University of Illinois professor of microbiology and principal investigator in the study. “One way that H. pylori promote their survival is by releasing a toxin that acts upon cells making up the stomach lining, as well as immune cells whose job is to recognize and eliminate invading microbial pathogens.
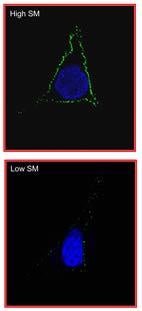
“By altering the properties of human cells to create a more suitable niche for colonization, the VacA toxin helps H. pylori gain a foothold in the stomach and survive over the long-term, which may be a person’s entire lifetime,” he explains.
Blanke’s team has now provided direct insight into how this toxin manages to get into stomach cells. The membranes that surround our cells are designed to keep things out, so the primary way toxins penetrate this barrier is by hijacking membrane surface receptors as they make their normal journey into the interior of cells.
The U of I team discovered that, in this case, the VacA toxin binds to a receptor called sphingomyelin, which is an important and abundant lipid on the surface of human stomach cells. Sphingomyelin has the ability to cluster into what almost looks like microscopic rafts—raised platforms on the cell surface. Blanke’s team found that the toxin binds to and enters the cell by way of these rafts.
“The platforms serve as entry portals for the toxin into the cell,” he says. Once inside, the toxin does its work, changing the cells in such a way that promotes survival of H. pylori bacteria in the stomach.
By identifying the receptors that the toxins use to get into the cell, drug makers can target the receptors for the development of new drugs to block the action of toxins upon cells, Blanke points out. By blocking the binding of VacA toxins to cellular receptors, these drugs can potentially make the stomach a less friendly place for the H. pylori bacteria.
The results of the U of I study were recently published in PLoS Pathogens, a leading journal in the infectious disease field. They were also highlighted in Microbe, the American Society for Microbiology’s monthly news publication, which features advances of special interest across all areas of microbiology, including infectious diseases.


