They have been called “the cockroaches of bacteria” by the head of the Centers for Disease Control and Prevention. Certain strains of the Staphylococcus aureus bacteria, better known as “staph,” can be as resilient and as elusive as the common cockroach.
To battle these microscopic bugs, LAS biochemists have genetically engineered a protein that can neutralize several toxins emitted by staph bacteria. Breakthroughs such as this take on an even greater urgency as new waves of staph bacteria build resistance to antibiotics.
Antibiotic-resistant staph, once mainly isolated to hospitals, has moved into the community in recent years, says David Kranz, a U of I biochemistry professor specializing in immunology. In fact, the CDCP estimates there are about 94,000 cases of infections by antibiotic-resistant staph in the United States each year, with more than 18,000 deaths—slightly higher than U.S. deaths from AIDS.
A U of I team, led by Kranz, has engineered a protein that can neutralize Staphylococcus enterotoxin B (SEB), one of the primary toxins emitted by staph bacteria. In their animal tests, conducted with Pat Schlievert, a world expert on staph from the University of Minnesota, the engineered protein has cured all infected rabbits. The next step is to conduct clinical trials with humans.
“It’s a pretty remarkable agent,” Kranz says.
In addition to their success with SEB, Kranz’s team of 15 researchers has engineered proteins that may be able to neutralize two other potentially deadly staph toxins—Staphyloccocus enterotoxin C (SEC) and toxic shock syndrome toxin 1 (TSST1). TSST1 is most infamous for the rash of toxic shock syndrome deaths related to the use of certain tampons in the early 1980s.
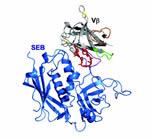
All three toxins—SEB, SEC, and TSST1—act in much the same way, he explains. The toxins bind to white blood cells, known as T cells, triggering a massive immune response, in which large amounts of cytokines are released into the body. High levels of cytokines can damage body organs, sometimes leading to death.
What Kranz’s team has done is engineer proteins that bind tightly to these enterotoxins. Once the protein binds to an enterotoxin, the entire complex can be safely cleared from the body, such as through the kidneys. However, the engineered proteins that they developed to battle SEC and TSST1 are not quite as far along as the one targeting SEB; they have yet to be tested with animals, says Kranz.
Kranz and Schlievert’s tests also have found that staph toxins can do damage to lungs when infections follow the flu. But their treatment can actually help to prevent the lung problems. In addition to the clinical importance of this research, the work on staph is vital in biological warfare defense. SEBs, SECs, and TSST1’s all could potentially be used in a terrorist attack. That’s why the research is being funded as part of a biodefense initiative with the National Institutes of Health.
Kranz says their treatments could be injected into the body to get the toxin inhibitors into a person’s system as quickly as possible. Kranz and Schlievert are also considering a spray to deliver the inhibitors in those cases in which the staph toxin targets the lungs.
Another class of diseases caused by staph includes skin diseases. In these cases, the logical way to deliver the inhibitors would be through a cream or ointment. But whatever the delivery system, he says their toxin inhibitors would never be used alone.
“They would be used in combination with antibiotics,” he explains. “Antibiotics are used to eliminate the bacteria, but they do not eliminate the toxin created by the bacteria. Our inhibitors would get at the toxins.”