A new study led by chemists at the University of Illinois Urbana-Champaign brings fresh insight into the development of semiconductor materials that can do things their traditional silicon counterparts cannot – harness the power of chirality, a non-superimposable mirror image.
Chirality is one of nature’s strategies used to build complexity into structures, with the DNA double helix perhaps being the most recognized example – two molecule chains connected by a molecular “backbone” and twisted to the right.
In nature, chiral molecules, like proteins, funnel electricity very efficiently by selectively transporting electrons of the same spin direction.
Researchers have been working for decades to mimic nature’s chirality in synthetic molecules. A new study, led by chemical and biomolecular chemistry professor Ying Diao, investigates how well various modifications to a non-chiral polymer called DPP-T4 can be used to form chiral helical structures in polymer-based semiconductor materials. Potential applications include solar cells that function like leaves, computers that use quantum states of electrons to compute more efficiently and new imaging techniques that capture three-dimensional information rather than 2D, to name a few.
The study findings are reported in the journal ACS Central Science.

“We started by thinking that making small tweaks to the structure of the DPP-T4 molecule – achieved by adding or changing the atoms connected to the backbone – would alter the torsion, or twist of the structure, and induce chirality,” Diao said. “However, we quickly discovered that things were not that simple.”
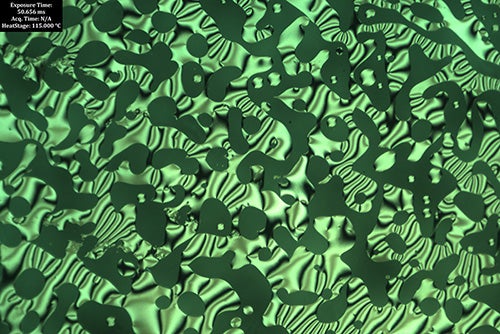
Using X-ray scattering and imagining, the team found that their “slight tweaks” caused major changes in the phases of the material.
“What we observed is a sort of Goldilocks effect,” Diao said. “Usually, the molecules assemble like a twisted wire, but suddenly, when we twist the molecule to a critical torsion, they started to assemble into new mesophases in the form of flat plates or sheets. By testing to see how well these structures could bend polarized light – a test for chirality – we were surprised to discover that the sheets can also twist into cohesive chiral structures.”
The team’s findings illuminate the fact that not all polymers will behave similarly when tweaked in an effort to mimic the efficient electron transport in chiral structures. The study reports that it is critical to not overlook the complex mesophase structures formed to discover unknown phases that can lead to optical, electronic and mechanical properties unimagined before.
Purdue University professor Jianguo Mei and graduate student Xuyi Luo synthesized the polymers used in this study. Diao also is affiliated with materials science and engineering, chemistry, the Materials Research Laboratory and the Beckman Institute for Advanced Science and Technology at Illinois.
The Office of Naval Research, the Air Force Office of Scientific Research, the National Science Foundation and the U.S. Department of Energy supported this research.