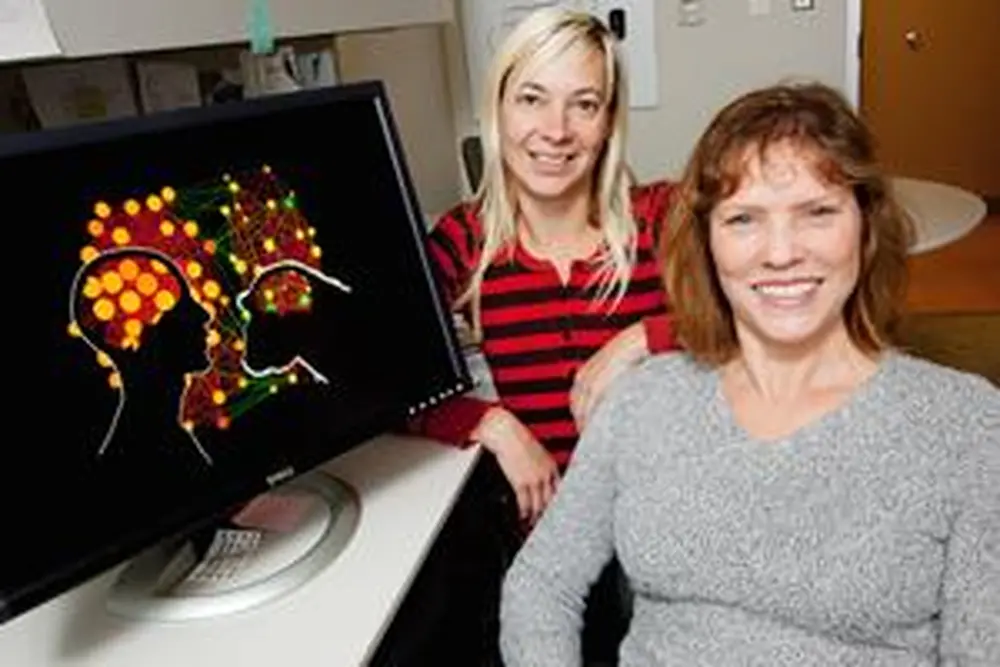
Size isn’t all that matters when it comes to the brains of chimpanzees and humans.
Humans have significantly larger brains than chimps, especially in the forebrain region. But LAS researchers have found that gene expression in the brain—the way genetic information is used—sets humans apart from chimps in even more dramatic ways.
Chimps and humans share most of the same genes, says Lisa Stubbs, a University of Illinois cell and developmental biology professor. “But we’re seeing that the number of genes with expression differences is large—surprisingly large,” she says.
Stubbs, who helped to analyze the sequencing of both the human and mouse genomes, has long been interested in the genetic differences among species. She says some genes are unique to a species, such as those that encode olfactory receptors in the nose. That is why many animals, such as dogs and mice, can detect odors that humans cannot even imagine being able to smell.
In the chimp study, however, she examined genes in the brain that humans and chimps have in common. And she found that the differences lay in the way the genes are expressed. Stubbs, along with postdoctoral researcher Katja Nowick and computational biologist Tim Gernat, analyzed gene expression data collected in Germany from the tissues of five chimpanzees and six humans. Then, using innovative computational methods developed by Norwegian researcher Eivind Almaas, they were able to see precisely how humans and chimps orchestrate brain gene expression in significantly different ways.
The key to the differences can be found in a group of genes known as transcription factors, or TFs. A single TF gene can control hundreds of other genes. Therefore, a change in one TF gene can have a cascading effect by altering the expression of hundreds of genes under its control.
In particular, Stubbs’s team zeroed in on a group of TF genes that are quite amenable to changing from species to species—KRAB zinc finger genes. They observed how groups of these genes worked together, along with other types of TFs, to either active or repress genes.
The team found a cluster, or network, of TFs that work together to control genes responsible for energy metabolism in the brain, which is much different in humans than in chimps, Stubbs says.
In addition, because human forebrains are bigger, human neurons must stretch long distances to be able to connect with other brain cells. This, in turn, means that the mechanisms needed to transport proteins along the extended neurons are more highly expressed in human brain tissue than in chimp brains. Changes in the organization and expression of TF genes make these metabolism marvels possible.
“Our findings suggest that a lot of the differences in gene expression between human and chimp brains are coordinated by these TF genes,” Stubbs says.
All of this research not only broadens the base of knowledge of how the human brain operates; it also sheds light on brain disorders that can result when the system goes wrong. For example, the TF network also regulates a system in the brain that prevents “free radical” oxygen—a byproduct of increased metabolism—from damaging cells. The breakdown of this system has been implicated in many brain disorders, including Parkinson’s and Alzheimer’s.
“The function of most of the 2,000 human TFs is unknown,” Stubbs says. “For example when we started, KRAB zinc finger genes were almost completely unstudied. Even today, of the 420 genes of this type, only 20 or 30 have known functions. So we still have a lot to do.”


