Chemists at the University of Illinois have successfully produced fuels using water, carbon dioxide, and visible light through artificial photosynthesis. By converting carbon dioxide into more complex molecules like propane, green energy technology is now one step closer to using excess carbon dioxide to store solar energy – in the form of chemical bonds – for use when the sun is not shining and in times of peak demand.
Plants use sunlight to drive chemical reactions between water and carbon dioxide to create and store solar energy in the form of energy-dense glucose. In the new study, the researchers developed an artificial process that uses the same green light portion of the visible light spectrum used by plants during natural photosynthesis to convert carbon dioxide and water into fuel, in conjunction with electron-rich gold nanoparticles that serve as a catalyst. The new findings are published in the journal Nature Communications.
“The goal here is to produce complex, liquefiable hydrocarbons from excess carbon dioxide and other sustainable resources such as sunlight,” said Prashant Jain, a chemistry professor and co-author of the study. “Liquid fuels are ideal because they are easier, safer, and more economical to transport than gas and, because they are made from long-chain molecules, contain more bonds – meaning they pack energy more densely.”
In Jain’s lab, Sungju Yu, a postdoctoral researcher and first author of the study, uses metal catalysts to absorb green light and transfer electrons and protons needed for chemical reactions between carbon dioxide and water – filling the role of the pigment chlorophyll in natural photosynthesis.
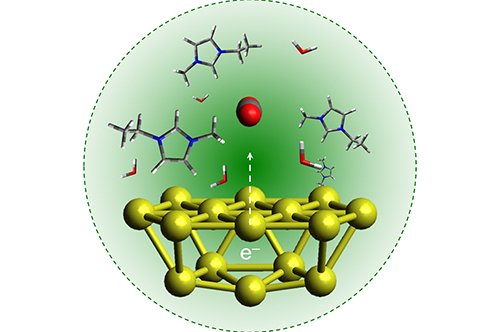
Gold nanoparticles work particularly well as a catalyst, Jain said, because their surfaces interact favorably with the CO2 molecules, are efficient at absorbing light, and do not break down or degrade like other metals that can tarnish easily.
There are several ways in which the energy stored in bonds of the hydrocarbon fuel is freed. However, the easy conventional method of combustion ends up producing more carbon dioxide – which is counterproductive to the notion of harvesting and storing solar energy in the first place, Jain said.
“There are other, more unconventional potential uses from the hydrocarbons created from this process,” he said. “They could be used to power fuel cells for producing electrical current and voltage. There are labs across the world trying to figure out how the hydrocarbon-to-electricity conversion can be conducted efficiently,” Jain said.
As exciting as the development of this carbon dioxide-to-liquid fuel may be for green energy technology, the researchers acknowledge that Jain’s artificial photosynthesis process is nowhere near as efficient as it is in plants.
“We need to learn how to tune the catalyst to increase the efficiency of the chemical reactions,” he said. “Then we can start the hard work of determining how to go about scaling up the process. And, like any unconventional energy technology, there will be many economic feasibility questions to be answered, as well.”
The Energy and Biosciences Institute, through the EBI-Shell program, supported this research.


