Clinical testing is underway for a potentially groundbreaking new treatment for cystic fibrosis. Pioneered by scientists at the University of Illinois Urbana-Champaign and the University of Iowa in partnership with the spin-out biotechnology company cystetic Medicines, this promising inhalable molecular prosthetic is intended to improve lung function in people with CF who cannot benefit from current therapies.
The launch of this clinical trial is an important step in a joint public-private effort to develop a safe and effective treatment for everyone with CF, a progressive genetic disorder characterized by persistent lung infections that can cause severe damage over time.
“We're hopeful that for those who have held their breath for far too long, this could be a first opportunity to regain ion-channel-like function in the airway and thereby address CF at its roots,” said Martin D. Burke, the May and Ving Lee Professor for Chemical Innovation, who leads the research team in collaboration with scientists from the University of Iowa.
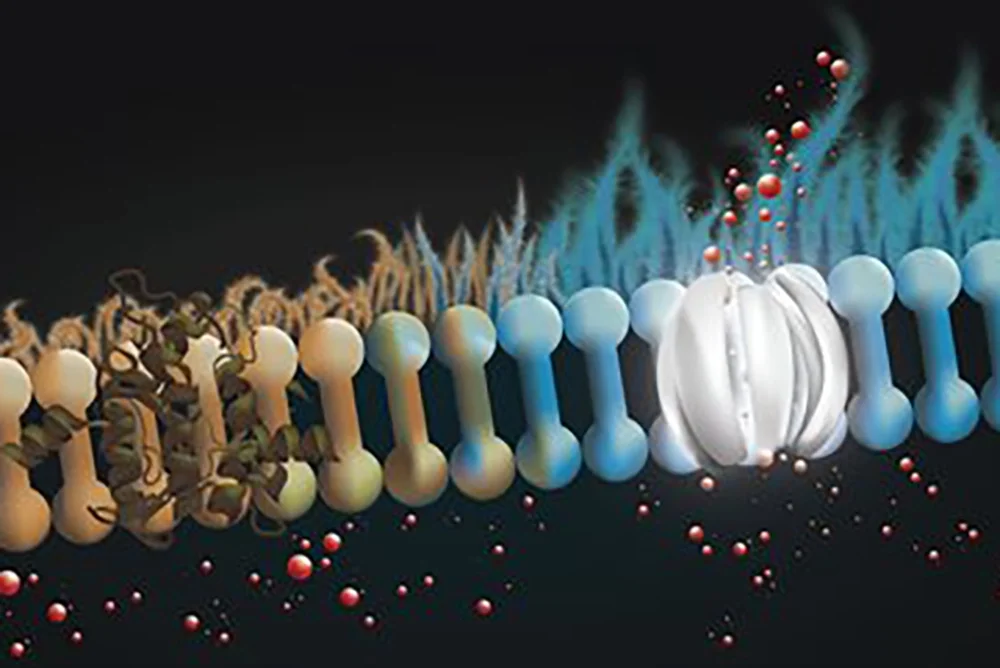
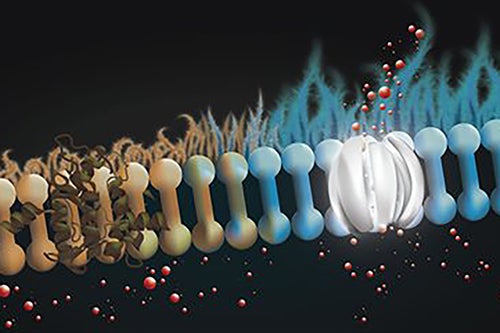
The first human volunteers in New Zealand recently began taking the new inhaled drug called CM001 (also known as amphotericin B cystetic for inhalation or ABCI), a molecular prosthetic that "stands in" for missing or dysfunctional protein channels and is intended to restore more normal lung function in patients with cystic fibrosis. The approach combines a novel inhaled dry powder formulation to directly target the lungs and allow for more consistent dosing.
“With this method, people with CF could directly deliver this molecular prosthetic to their lungs where they need it most, hopefully increasing its efficacy and safety,” Burke said. The ongoing clinical trial is intended to evaluate the new drug’s safety, tolerability, and movement through the body.
Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene responsible for producing the protein that helps regulate the balance of anions and fluid in the lining of the lungs and other organs. In the lungs, this dysfunction results in the formation of thick, sticky mucus on the lung lining that makes it difficult to breathe and causes frequent lung infections. About 90 percent of people with CF produce CFTR protein that doesn’t work properly. For these individuals, a class of drugs called CFTR modulators can often restore protein-channel function and result in improved breathing. But treatments have been extremely limited for patients whose bodies produce little or no CFTR protein. Burke and his team are optimistic that CM001 can act as a prosthetic at the molecular scale, replacing the missing protein and restoring ion channel function.
“The first impact we're hoping to achieve is by partnering with that final 10 percent of the CF community that cannot benefit from modulators to determine if we can provide benefit in a way that would address this fundamental defect,” Burke said.
While the new drug CM001 may be a game-changer for patients who are not treatable with CFTR regulators, other CF patients could benefit as well.
“The concept of molecular prosthetics has the potential to alter the treatment landscape for cystic fibrosis in a profound way; in theory, it should work for all people dealing with the disease, regardless of the type of mutation they present,” said Jeffry Weers, chief technology officer of cystetic Medicines and an industry leader in the development of inhalable medicines.

Burke, who is a a professor of chemistry and of the Carle Illinois College of Medicine as well as a medical doctor, says the development of this potential new treatment is powered by a translational, multi-disciplinary approach to solving health care problems.
“We were able to get the all-star team together, and the whole mission of the company was to translate this basic science finding that happened here at Illinois and the University of Iowa into societal impact.” cystetic Medicines co-founder Dr. Michael Welsh, a professor at the Roy J. and Lucilla A Carver College of Medicine at the University of Iowa, is a leading expert on cystic fibrosis. His lab was instrumental in collaborating with Burke’s lab at UIUC throughout the research process, including foundational research studies.
“The collaboration between UIUC and the University of Iowa has been key in developing the molecular prosthetic approach to treat cystic fibrosis,” said Ian Thornell, a research assistant professor in the Division of Pulmonary, Critical Care and Occupational Medicine in the Department of Internal Medicine at the University of Iowa. He is also one of the key research collaborators. “UIUC has employed cutting-edge chemistry to design and test different molecular prosthetics, while the University of Iowa is one of nine basic science CF research hubs designated by the Cystic Fibrosis Foundation. The merging of technology developed at UIUC and Iowa’s CF expertise contributed to the success within the pre-clinical phase of this trial.”
People with CF have played a crucial role in earlier testing of the new treatment, said Agnieszka Lewandowska, a senior research scientist and a member of Burke’s lab. “We are grateful for the willingness of the CF community that provided cells through the University of Iowa’s Cystic Fibrosis Research Center that ultimately brought these compounds to clinical trial, Lewandowska said. “In collaboration with laboratories of Mike Welsh and Ian Thornell at the University of Iowa, we were able to demonstrate that ABCI, developed in partnership with cystetic Medicines and tested at UIUC, restores ion channel function to cells from people with CF.”
The work to develop a new treatment for the broad range of CF patients has also garnered financial support from both the public and private sectors, including a $32 million investment from Deerfield Management and support from Illinois Ventures. The non-profit Emily’s Entourage also supported early-stage research in the Burke and Welsh labs with grant funding.
Burke’s ambitions for the future impact of molecular prosthetics extend beyond CF treatments to other diseases and conditions.
“Cystic fibrosis is one of hundreds of diseases that currently remain incurable because they're caused by loss of protein function,” Burke said. “The hope is that if we can succeed in CF, this molecular prosthetics approach could become a general way to treat diseases caused by loss of protein function.”
He also expects innovators at the world’s first engineering-based college of medicine will play an important role in developing new molecular prosthetics.
“There's a whole field around the intersection of engineering and prosthetic limbs. We’re now engineering prosthetics at the molecular scale. The whole goal of the technology that we're now building out at the Molecule Maker Lab at the Beckman Institute here at UIUC is to bring everybody into this exciting new space, including the brilliant physician-innovators at the Carle Illinois College of Medicine,” Burke said.
Results of the clinical trial are expected in 2024.


